Recruiting Participants
If you can’t get participants recruited for a trial or if they drop out, then that is the end of the study. Recruiting participants for the trial was a mammoth task and initially the researchers were unsure about how best to do this. They considered visiting schools and talking to students, but eventually it was decided to recruit via Facebook. There was a huge amount of interest and a large number of people (600) responded. Of these, over 300 people were spoken to for further testing.
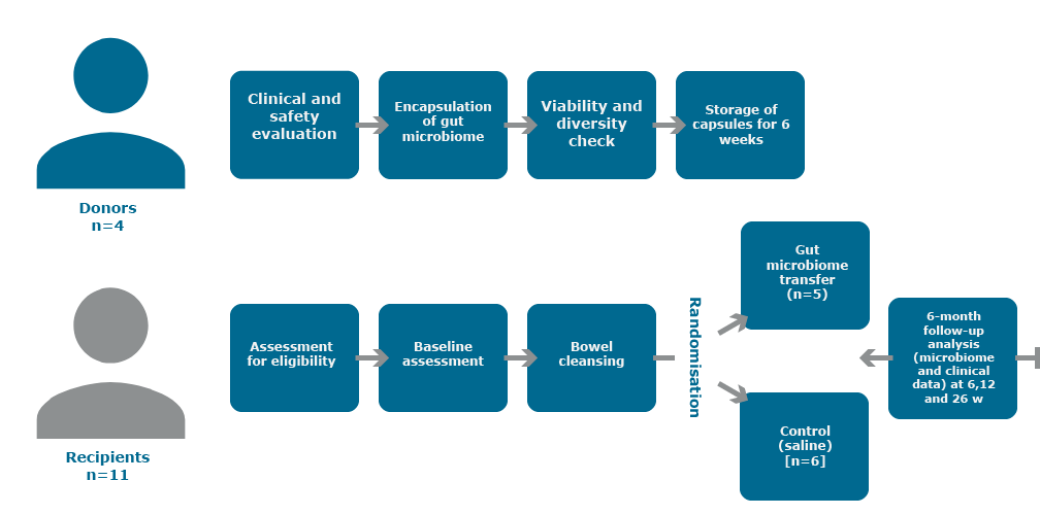
Potential participants and their caregivers were given an information sheet which told them what would happen in the trial and what they would be required to do if they agreed to take part. Eight young people were wanted for the pilot trial - four men and four women. However, eight young women were chosen, as only women responded.
The Donors
Faecal matter was taken from lean, physically active donors. One donor stool (faeces) would produce enough material for two same-sex recipients. Potential donors were screened to make sure they were free of infectious and chronic diseases and drug use (including nicotine and alcohol). These procedures were similar to those used for blood donation in New Zealand. Donors were interviewed to make sure they had not been exposed to other risk factors such as recent travel to high-risk countries. They were tested for leanness (low BMI) and for high levels of physical fitness and activity.
Of the 21 donors that volunteered for the pilot trial, only four passed the screening stage.
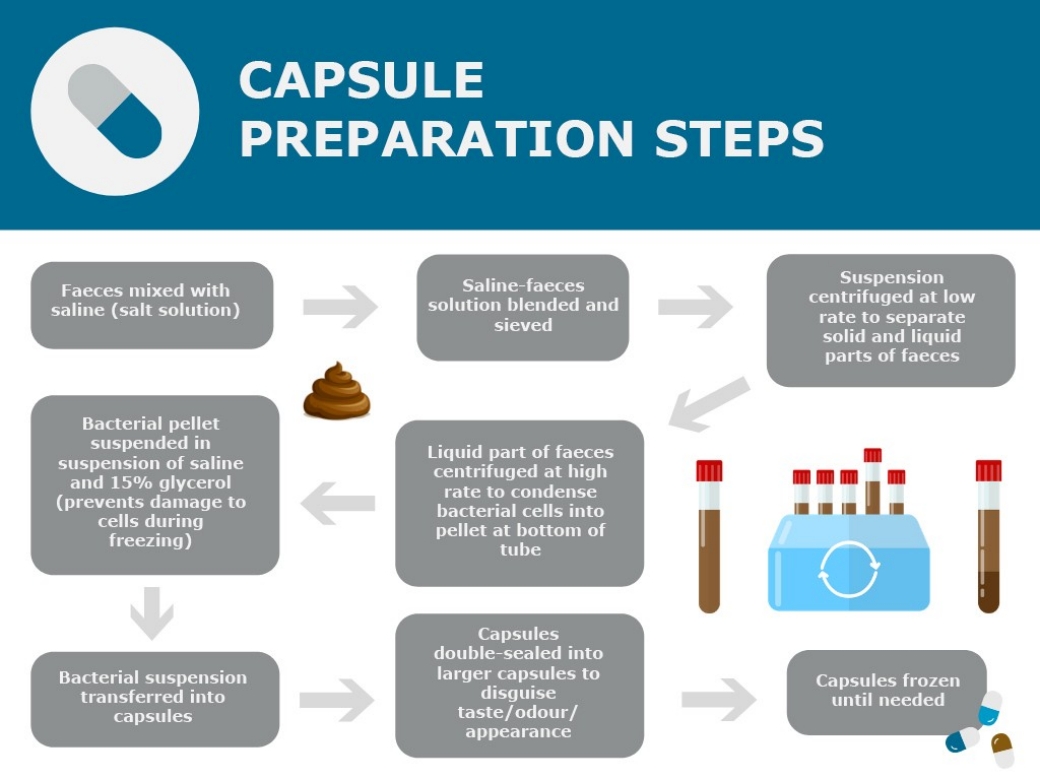
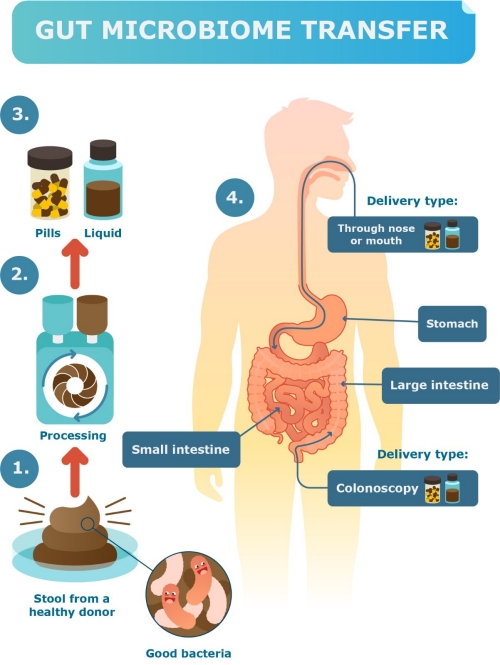
To collect the faeces, donors would come in to the Liggins Institute on a regular basis, about once a month. They would have something to eat, maybe drink a coffee, and then hopefully, they would be able to produce a stool sample. This sample was processed into capsules immediately. The donors’ stool samples were checked at 6, 12 and 26 weeks to ensure their gut microbiomes remained stable.