Free Radicals, Anti‐Oxidants and Oxidative Stress
In an attempt to find out why these changes were occurring, the science team measured the levels of a number of different markers that can indicate oxidative stress.
Oxidative stress is the term used to describe damage to cells, tissues and organs that is caused by a group of chemicals known as reactive oxygen species. These are chemicals such as free radicals or peroxidises which are produced as a result of the metabolism of oxygen and therefore exists in all aerobic species. Free radicals are highly reactive because they have at least one unpaired electron. The level of oxidative stress is determined by the balance between the rate at which free radicals cause damage and the rate at which anti‐oxidants repair damage.
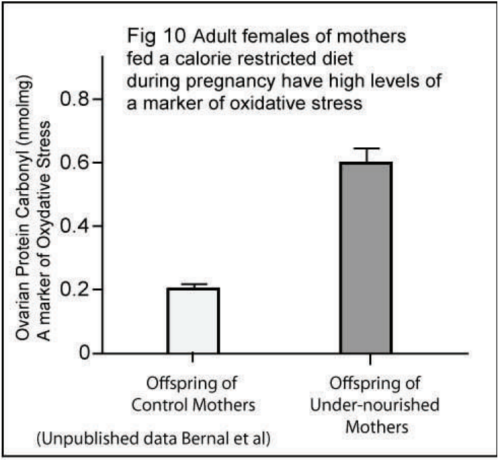
The science team found that the offspring of undernourished mothers had high levels of a molecule called Protein Carbonyl, a marker of oxidative stress, in their ovaries (Fig 10). They also found that the offspring of both undernourished and high-fat-fed mothers had altered levels of an enzyme that regulates levels of hydrogen peroxide - a chemical that is known to cause oxidative stress.
Developmental Programming: The Predictive Adaptive Response Theory
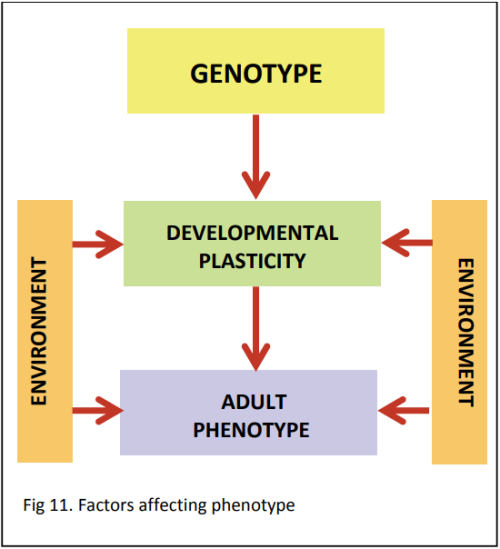
The prenatal environment is having an effect on adult phenotype. Professor Sir Peter Gluckman of the Liggins Institute and Professor Mark Hanson of the University of Southampton have proposed that the effects that scientists have observed are due to a mismatch between the environment that the fetus experiences in the womb and the environment that the child experiences once born. In the womb, the fetus has experienced “hard times” (i.e. there is not enough food) and will make a series of metabolic adaptations in order to survive. As a result of this, the fetus is well adapted for a life of under-nutrition. When it is born into a world where there is plenty of food, the child is not well adapted. This can result in a series of potential problems including obesity, diabetes, heart disease and early puberty.
Predictive Adaptive Responses
We know from examples in nature that some animals are capable of predicting their adult environment and adjusting their phenotype during development to ensure they are well adapted for the predicted adult environment, improving survival. These animals demonstrate a PREDICTIVE ADAPTIVE RESPONSE. An example of this is the meadow vole, a small animal (similar to a mouse) found in Alaska, Canada and the northern United States. With a life span of around six months, the meadow vole will experience either summer or winter conditions in its life - not both. Coat thickness is determined before birth by maternal signals (the hormone melatonin), related to whether the day length is shortening or lengthening. Meadow voles born at a time when they will live through winter (short days) develop a thick coat, whereas those born to live during summer (long days) will develop a thin coat. This is an example of DEVELOPMENTAL PLASTICITY.
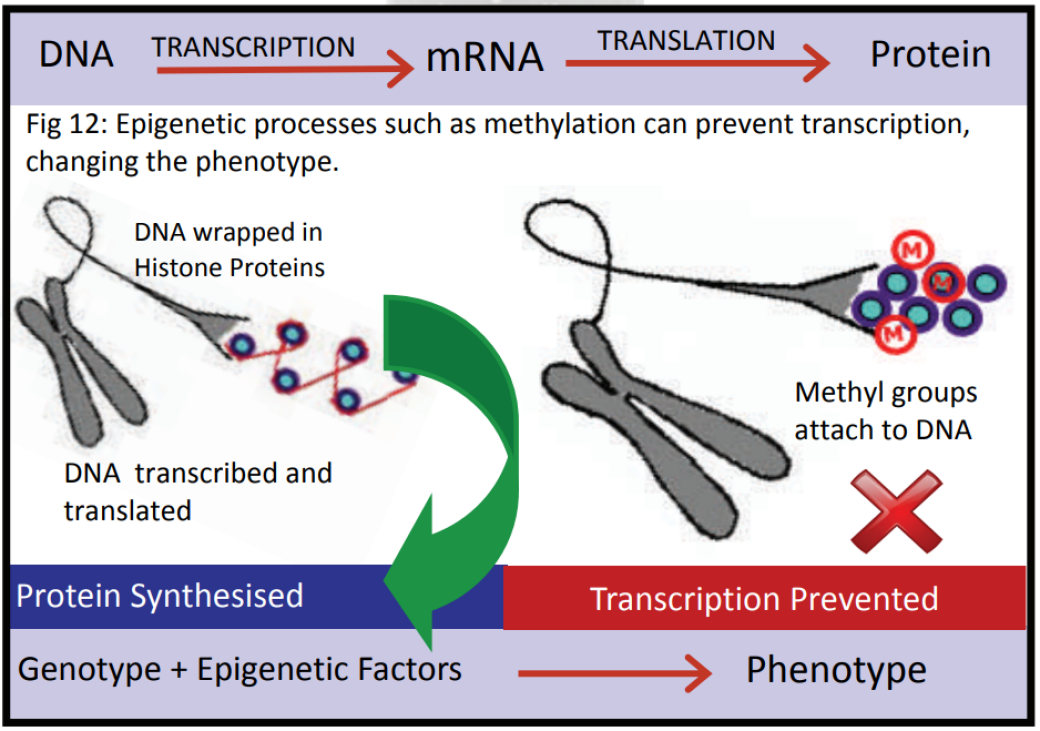
The phenotype is being determined by gene‐environment interactions, determining which genes are turned on to produce the phenotype most suitable for the predicted environment.