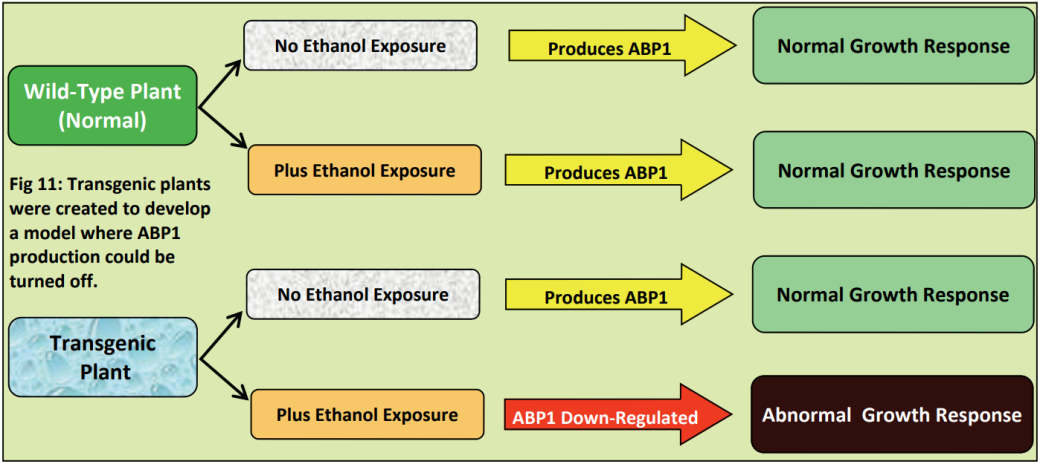
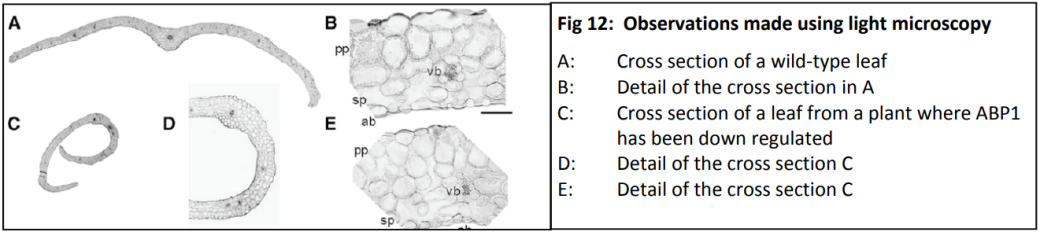
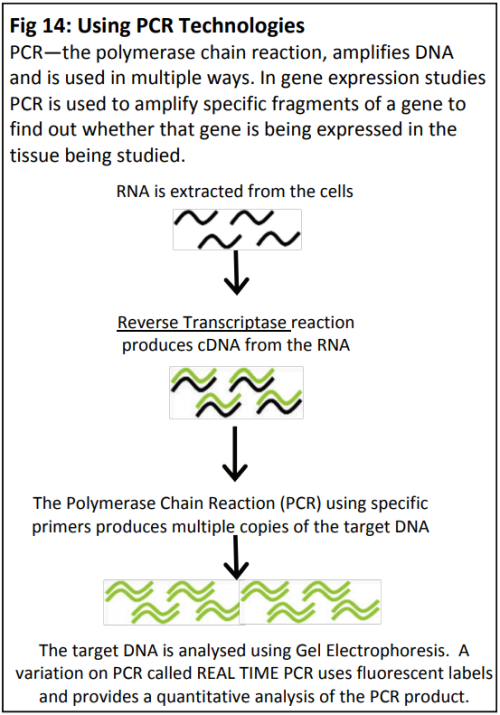
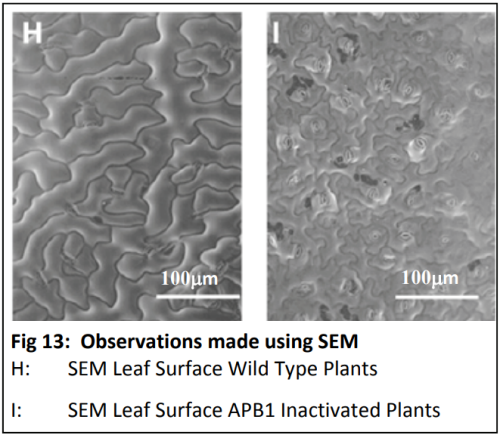
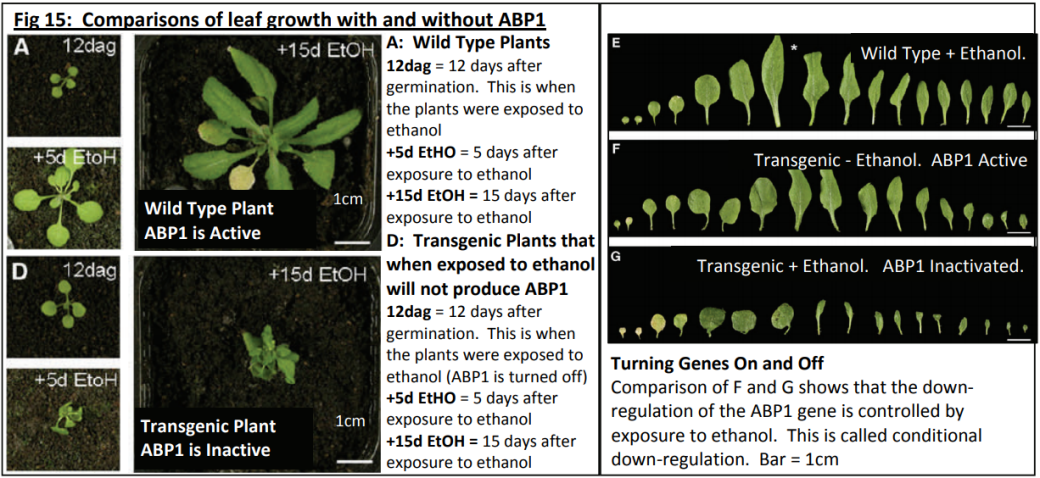
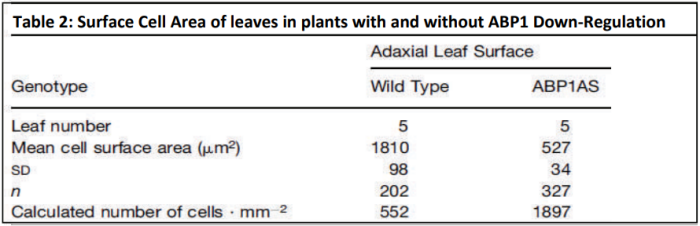
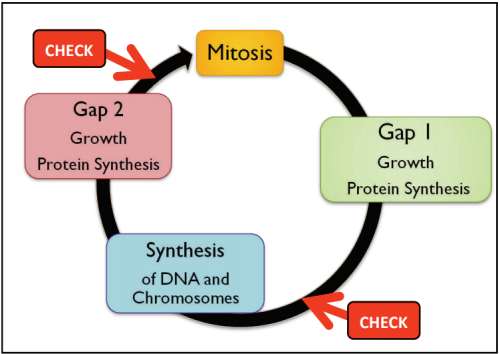
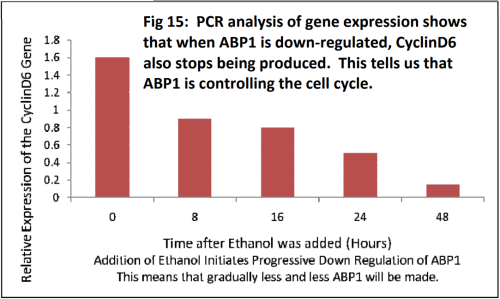
Dr Karine David is group leader within the Plant Molecular Sciences Laboratory at The University of Auckland. This lab has a number of groups looking into different aspects of plant growth and offers excellent training opportunities for young scientists, some of which involve work with Plant and Food Research. Karine’s team are contributing to the worldwide quest for scientific knowledge about HOW auxins work. They are specifically trying to find out more about how the cell can tell that the auxin is there - and then how does the cell “decide” to respond?
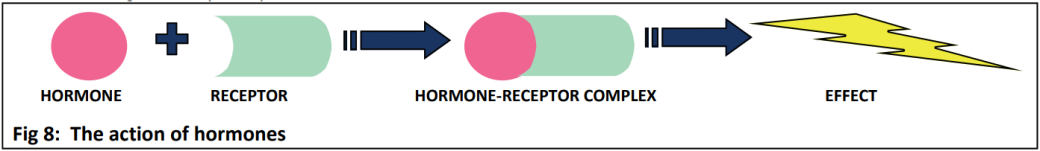
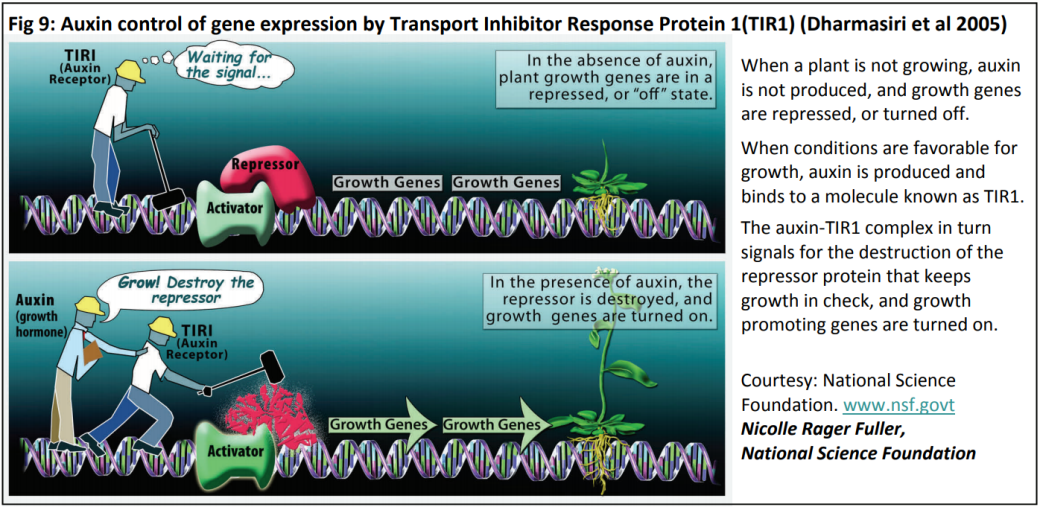
As a hormone, auxin will have one (or more) receptor(s) which must recognise the hormone and bind to it, enabling the hormone to act in the cell (see Fig 8). In the case of auxin, that action involves turning on genes that enable plant growth. The fact that auxin has a different effect in different situations (e.g. roots vs shoots) makes understanding this very complex.