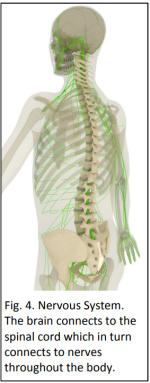
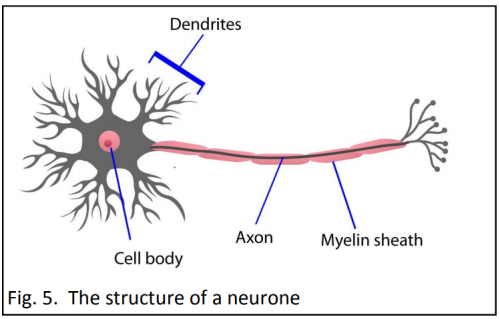
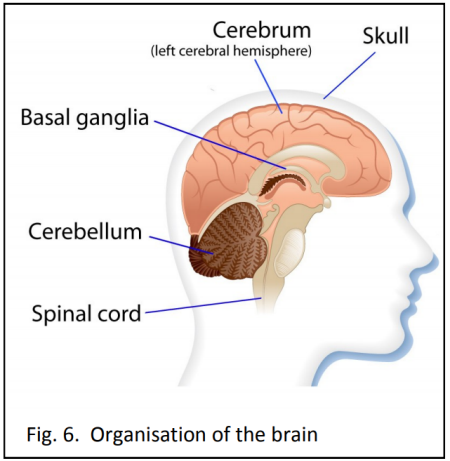
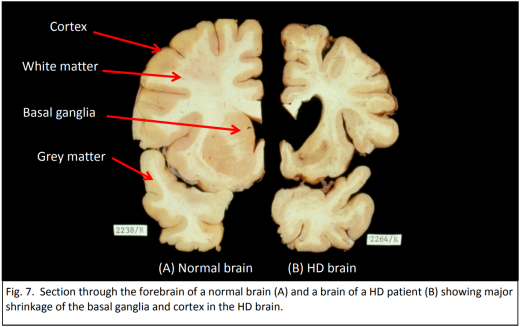
Strict conditions surround genetic testing. In New Zealand only people over 18 years of age are eligible, and they must be counselled before and after the test. Less than five percent of potential carriers elect to be tested, mostly because there is no cure.
Pre-implantation genetic diagnosis – where embryos created by IVF can be tested before a genetic disease before being implanted, is also available, but in New Zealand it is publicly funded only in a limited way, and usually if the at-risk parent has been tested first. Fetuses can also be tested, giving parents the option to terminate the pregnancy if the baby is found to be a carrier.
Genetic Fitness
HD is found in about 5–7 individuals per 100,000, although there are rare areas with a much higher prevalence. Amongst Asian and African peoples the prevalence is much lower; in Japan, for example, it is only 0.5 per 100,000.
There is no evidence for the HD mutation conferring any fitness on carriers: in other words, on average they have the same number of children as non-carriers. A mutated IT15 gene does nothing helpful. However, because its deleterious effects generally appear after carriers have had their children, it survives. Mutations whose effects take hold after child-bearing age have quite different patterns of allele survival to mutations whose effects appear earlier in life.
Some gene mutations have no effect at all on which amino acid is transcribed, so are silent, having no observable effect on the phenotype. Some mutations, however, do offer a selective advantage to their carriers. Sickle cell anaemia is one example of this. People who are heterozygous for the gene that makes their red blood cells sickle-shaped are more resistant to the effects of malaria. The sickle cell mutation is therefore relatively common in tropical countries. This selective advantage increases the likelihood of the allele surviving in the population. No such selective advantage can be identified for the Huntingtin allele.
Apart from racial heritage, the only factor that is identifiable in the patterns of Huntingtin alleles in different populations is that of founder effect. Where a population has established from a small gene pool in which the mutated gene was present, it is found in higher numbers in that population. One such population is found in Tasmania.
One Gene Mutation, but a Variable Disease
Once, it was hoped that the clear single-gene nature of HD would mean that a cure would be reasonably achievable, and that the knowledge gained would help combat other diseases. However, it is not as simple as it once seemed: although it is a single gene mutation, variation is present in both the genotype and the phenotype of HD. Understanding this variation is an important part of the process of finding answers in the HD puzzle.
In some cases, variation in the phenotype can be linked to variation in the genotype. This forms predictable patterns. In other cases, variation in the phenotype bears no relationship to variation in the genotype. When this happens scientists need to look for alternative reasons for this variation.
Phenotype Variation Caused by Genotype Variation
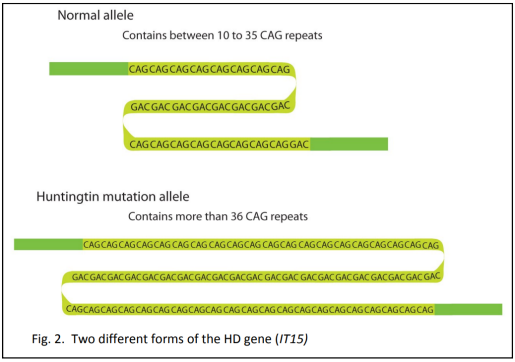
People with HD do not all have the same number of CAG repeats in their gene. The differing number of repeats is linked to two types of variation that are seen in the disease symptoms (the phenotype): the onset of disease and how quickly it progresses.
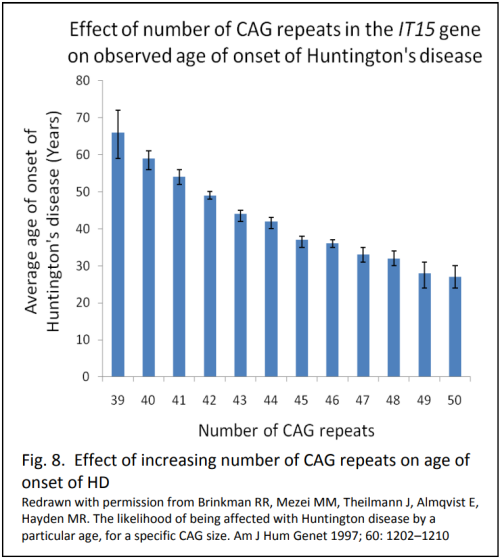
One aspect of phenotype variation that can be partially explained by genotype variation is the age of onset of the disease. Figure 8 shows that the longer the length of the chain of CAG repeats in the gene, the earlier the age of disease onset. When the chain is long enough, the disease manifests in children as young as one year of age. (In children the disease has different features: lack of movement, a rigid body and seizures). Sixty percent of the variability in age of onset is explained by length of CAG repeat, with other unknown factors contributing to the remaining 40%.
The age of onset of the disease can decrease with each successive generation. This can be accounted for by known instability of the IT15 gene in the testicles (where sperm are formed). In cases of 28 CAG repeats or more, the number of repeats is prone to increase during sperm formation. In this way, when genes are passed through the generations by males, chain length tends to increase. Therefore, in successive generations, the age of onset becomes increasingly younger. This is known as genetic anticipation.
This instability of the gene in the testicles also accounts for the rare spontaneous mutations. After some testicular hot housing, a previously normal but borderline number of 35 repeats can increase sufficiently to cause HD. People from white European races have higher frequency of huntingtin alleles with 28–35 repeats, and are therefore more vulnerable to instability during spermatogenesis. If the mutation occurs during spermatogenesis, it is a new or de novo mutation.